Gut microbiota is a source of pathogens in immunocompromised patients. While most microbiota studies in patients with AML receiving induction chemotherapy have focused on the gut microbiota, the few on salivary microbiota suggest less severe dysbiosis. As the saliva does not have its own intrinsic microbiota and takes fractions of the microbiota from the different oral tissues it bathes, the extent to which the salivary microbiota reflects microbial changes in other oral sites is unknown. To fill this knowledge gap, we characterized the microbiota in longitudinal salivary, supragingival plaque, and fecal samples of AML patients receiving inpatient chemotherapy.
31 patients have been enrolled in this prospective single-center study. Baseline stool, saliva, and plaque samples were collected at baseline and repeated weekly until 5 weeks after starting chemotherapy or discharge from the hospital, whichever occurred first. Time was divided into 3 intervals: admission to day 10 of chemotherapy, days 11-20, and day 21 and later. 111 samples (stool: 39, saliva: 36, plaque: 36) from 16 patients have been sequenced to date. Samples underwent shotgun sequencing targeting 10M paired-end reads/sample. MetaPhlAn4 was used for taxonomic assignment. Alpha diversity was quantified by Shannon index and beta diversity by Bray-Curtis dissimilarity.
9 patients were male and 7 were female. Median age was 64 (range 34-82). 15 patients were newly diagnosed and 1 was relapsed/refractory. Chemotherapy regimens included CLAG-M (n=10), ATRA+ATO (n=3), Vyxeos (n=1), Venetoclax+Azacitidine (n=1), and intermediate-dose Ara-C (n=1). The 3 most frequently used antibacterial antibiotics were cefepime (n=14), IV vancomycin (n=12), and levofloxacin (n=11). Median hospitalization length was 19 days (range 6-31). Neutropenic fever occurred in 13 patients. Documented infections included bacteremia (n=8), skin/soft tissue (n=5), intestinal (n=4), urinary tract (n=4), and pneumonia (n=1). Four bacterial species isolated from blood cultures of patients with fever were detected by sequencing 1-2 weeks before bacteremia: Gemella Haemolysans in saliva, plaque, and stool, Streptococcus mitis in saliva and stool, Rothia mucilaginosa in saliva and plaque, and Citrobacter freundii in stool.
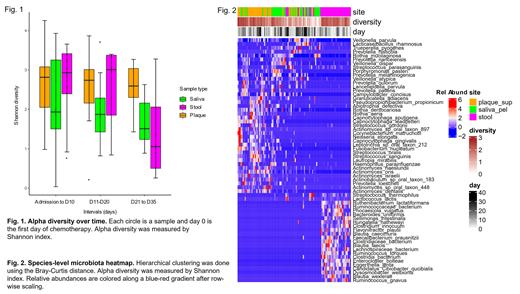
Microbiota diversity from the 3 sites showed markedly different dynamics ( Fig. 1). Plaque microbiota diversity remained stable throughout, with levels as high as fecal microbiota in intervals 1 and 2, and higher than both fecal and salivary microbiota in interval 3. Salivary microbiota diversity was the lowest among sites in intervals 1 and 2 and experienced only a slight reduction in interval 3. In contrast, fecal microbiota diversity was high compared to the other sites in intervals 1 and 2 but dropped in interval 3 to the lowest among all sites. With all 3 intervals combined, salivary microbiota had lower diversity (median 1.9, range 0.04-3.9) than plaque (median 2.7, 0.2-4.3; P=0.01) and fecal microbiota (median 2.9, 0.3-3.7; P=0.048). The 3 sites largely segregated on hierarchical clustering using species abundances ( Fig. 2). However, microbiota in several low-diversity samples from different sites in the same patient coalesced: Veillonella parvula and R. mucilaginosa were detected in all 3 sites, V. dispar in stool and plaque, and S. parasanguinis in stool and saliva. Each overlap was present in 5 or more patients.
This first shotgun sequencing analysis of longitudinal salivary, plaque, and fecal microbiota in AML patients offers several novel findings. First, plaque microbiota diversity is stable despite exposure to antibiotics, whereas fecal microbiota diversity is not. Second, while healthy adults show no overlap between oral and colonic microbiota, low-diversity fecal samples from AML patients contained species of their own oral microbiota, particularly at later time points. These data suggest ectopic colonization of the gut by oral flora. Future mechanistic studies of plaque biofilms may reveal community-protective mechanisms that sustain oral plaque microbial diversity and contribute oral flora to the gut. Mitigating gut dysbiosis in AML patients by manipulating plaque biofilms could be a novel approach to improve patient outcomes.
Disclosures
Rashidi:Seres Therapeutics, Ltd.: Consultancy. Appelbaum:2seventy bio: Research Funding. Percival:Astex, Ascentage, Abbvie, Biosight, BMS, Glycomimetics, Pfizer, Telios: Research Funding. Halpern:Abbie, Notable Labs, Agios: Consultancy; Imago Bioscience, Bayer, Gilead, Jazz, Incyte, Karyopharm Therapeutics, Disc Medicine: Research Funding. Walter:Abbvie, Adicet, Amphivena, BerGenBio, Bristol Myers Squibb, GlaxoSmithKline, Orum: Consultancy; ImmunoGen, Jura: Consultancy, Research Funding; Amgen, Aptevo, Celgene, Janssen, Jazz, MacroGenics, Pfizer: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal